The information contained herein is not for publication or distribution, directly or indirectly, in or into the United States. These written materials do not constitute an offer of securities for sale in the United States. The securities have not been and will not be registered under the U.S. Securities Act of 1933, as amended, and are not being offered or sold in or into the United States. The issue, exercise or sale of securities in the offering are subject to specific legal or regulatory restrictions in certain jurisdictions. The Company assumes no responsibility in the event there is a violation by any person of such restrictions.
**** A Finnish investment blogger and private investor Jarkko Aho (Random Walker) has made an individual company analysis of Aplagon Oy. The analysis can be found in the documents section at the bottom of this webbsite. Please notice that the analysis is only in Finnish. Please log in in order to see the whole investment material and the documents. ****
Investment information
- Type:
- Equity offering
- Invested:
- €2,227,944.00
- Equity offered:
- 8.22 – 16.47 %
- Price per share :
-
€24.00
min investment 35 shares
- Number of existing shares:
- 465,020
- Fully diluted shares:
- 512,277
- Pre-money valuation:
- €11,160,480.00
Our story
APAC – the first vascular injury targeting product for local prevention and treatment of occlusions of blood vessels
Aplagon is a Finland-based pharmaceutical development company developing APAC, the only vascular injury targeting product for local prevention and treatment of blood vessel occlusions.
We are now raising up to EUR 2.0 million in funding to demonstrate preliminary safety and efficacy of APAC (clinical proof-of-concept) in two indications – COVID-19 and hemodialysis access failure – in parallel in 2021-2022. Clinical proof-of-concept is the first major value inflection point in pharmaceutical development. Aplagon’s active lead investors and the management team will participate in the funding round with a combined investment of EUR 710,000.
Our goal is to find a global commercialisation partner for APAC after the planned clinical studies, providing an opportunity, in one transaction or stepwise, for an exit to the investors.
History
Aplagon has its origins in the research performed by Prof. Riitta Lassila’s group at Wihuri Research Institute in Helsinki, Finland on mast cell -derived heparin proteoglycans (HEP-PG). HEP-PGs reside among others in vascular tissue and act as the body’s own local vascular repair molecules to prevent blood clots at the vascular injury site (antithrombotics). Riitta and her group discovered that HEP-PGs act as potent antiplatelet agents in small blood vessels and occluded arteries. When HEP-PG structure is mimicked by linking together in a specified ratio two drugs that are in active clinical use today, unfractionated heparin (anticoagulant) and human serum albumin (important protein produced by liver), both antiplatelet (AP) and anticoagulant (AC) activity can be achieved. This molecule structure is able to prevent both forms of blood clot formation simultaneously. The discovery led eventually to APAC, a mimic of natural HEP-PGs, and setting up Aplagon to develop and commercialise APAC.
APAC has in addition to the prevention of blood clots another novel key feature – vascular injury targeting. This means that APAC homes (finds its way) where it is needed, to the sites of arterial injury where blood clots occur. Strong flow conditions prevail inside the arteries and currently used drugs are not able to efficiently stick to the damaged vessel wall site. APAC identifies the injured sites, attaches and stays there long enough to secure healing. Interestingly, APAC has also been shown to prevent acute kidney injuries after arterial occlusions.
The unique combination of the vascular injury targeting, and localised dual antiplatelet-anticoagulant activity enables a completely new, local treatment approach. Based on these mechanisms, there are many potential indications for APAC, but we are first targeting areas without approved or adequate therapies despite the medical need being high.
After 20 years of hard work since the initial HEP-PG discoveries, APAC is now about to enter clinical studies in COVID-19 and hemodialysis access failure, with results expected in the next 12-18 months. COVID-19 serves also as a stepping stone to a larger market of severe inflammation-induced coagulation disorders, a multi-billion euro market opportunity. We are also planning a clinical program in critical limb ischemia, the most advanced form of peripheral arterial disease, another large indication. There are no similar therapies available or in development for any of these indications. APAC’s functionalities are also well-suited for preventing arterial occlusions following vascular surgery and other vascular interventions, hemodialysis access surgery being one example.
We have a collaboration agreement in place with Cadila Pharmaceuticals Ltd, one of the leading pharmaceutical companies in India. The collaboration covers the initial clinical studies in both COVID-19 and hemodialysis access failure, enabling a highly cost-effective way of achieving preliminary efficacy and safety results in patients. Aplagon retains all the rights outside India.
Aplagon has raised to date €9.3 million in funding, of which €6.5 million through equity investments and €2.8 million through Business Finland (formerly Tekes) R&D-loans.
Why invest in Aplagon?
- APAC is a unique product with potential to provide a novel way of treatment in the target indications.
- APAC targets large, often multi-billion euro indications with significant unmet medical need.
- Aplagon’s goal is to demonstrate preliminary safety and efficacy of APAC (clinical proof-of-concept), the first major value inflection point in pharmaceuticals development, in two indications in parallel in the next 12-18 months.
- Aplagon’s aim is to make a global partnering deal after the preliminary clinical proof-of-concept has been demonstrated, providing the investors an opportunity for exit, in one transaction or stepwise.
- Aplagon has a collaboration agreement with Cadila Pharmaceuticals, a leading Indian pharmaceutical company. The collaboration provides the access to the resources of a large company and a highly cost-efficient way of generating clinical proof-of-concept for APAC.
- Aplagon has a highly experienced, international team and cost-efficient business model.
- Aplagon offers potential for an attractive investment return. Deal values for pharmaceutical products after clinical proof-of-concept can be very high, often in hundreds of millions of euros (see Section "Valuation").
- Aplagon’s active lead-investors, Jenny and Antti Wihuri Foundation, Gösta Serlachiuksen taidesäätiö and Innovestor Kasvurahasto I Ky, and the management team, Sakari Lassila, Riitta Lassila, Aki Prihti and Harry Holthöfer, will participate in the funding round with a combined investment of EUR 710,000.
What are blood vessel occlusions and what is their significance?
Blood is a constantly circulating fluid consisting of blood cells critical for life: plasma (protein-rich fluid), red blood cells, white blood cells, and platelets. Heart pumps oxygen-rich blood from lungs through the vascular system. Blood carries oxygen (red blood cells), takes care of body’s defense system (white blood cells), stops local bleeding (a process called hemostasis), and initiates wound healing (platelets) when necessary, and takes away waste materials (via liver, kidneys, lungs).
Thrombosis is the formation of an excessive blood clot (thrombus) inside a blood vessel, impairing or obstructing the blood flow. In an arterial thrombosis the target organ is deprived of oxygen, leading to infarction, a mode of cell death. In a venous thrombosis the return of blood circulation from peripheral organs is impaired. A blood clot that breaks free and begins to travel in circulation is known as an embolus, which may trigger thrombosis and organ failure at a site distant from its point of origin.
Thrombotic complications of cardiovascular disease and of cancer are a major cause for mortality. One in four people worldwide die from conditions caused by thrombosis.
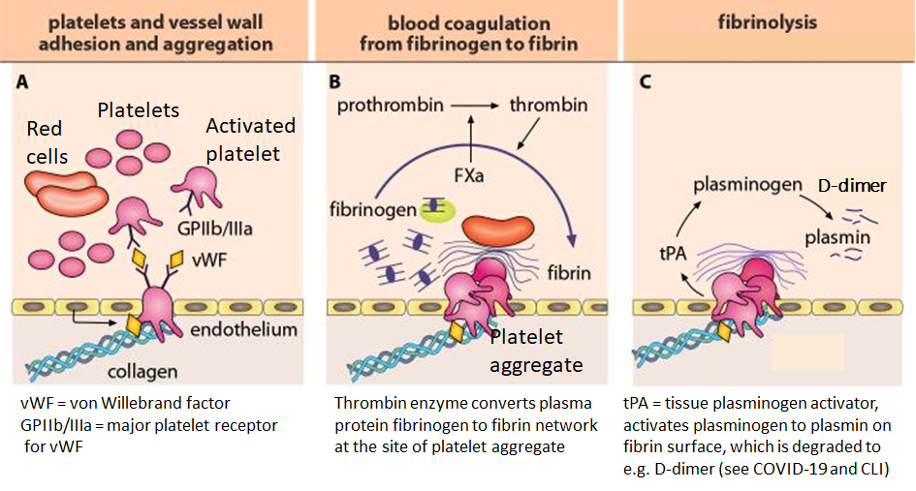 Lassila. Veritaudit, oppikirja, Blood diseases, textbook, Eds Porkka, Lassila, Remes, Savolainen, Duodecim, 2015.
Lassila. Veritaudit, oppikirja, Blood diseases, textbook, Eds Porkka, Lassila, Remes, Savolainen, Duodecim, 2015.
When a blood vessel is injured, hemostasis, the body’s repair mechanism to fix a wound, sets in. The body uses platelets and fibrin to seal the injury area to prevent blood loss and organ damage. This involves the interaction of blood cells and numerous proteins and enzymes, such as coagulation factors. The figure below shows the cascade from vascular injury to hemostatic plug formation (from vascular injury to the arrest of bleeding). An unnecessarily strong coagulation response would lead to a thrombus formation, occluding the blood vessel.
What is APAC?
Aplagon’s product APAC is a pharmaceutical compound composed of two widely used biological drugs, animal-derived unfractionated heparin (UFH) and human serum albumin (HSA). Specified number of UFH chains are coupled to HSA via a linker molecule.
APAC mimics mast-cell derived heparin proteoglycans (HEP-PG) and thereby body’s own defense and tissue repair mechanism.
HEP-PGs reside in vascular tissue and act as local vascular repair molecules and local antithrombotics (prevent clot formation at the vascular injury site).
Aplagon has studied several different APAC-conjugates. An optimised APAC-conjugate has been selected for clinical development.
What makes APAC unique?
How is APAC different from the clinical used heparins?
- Clinically used heparins, unfractionated heparin (UFH) and low-molecular weight heparin (LMWH), are widely used for systemic anticoagulation, i.e. treatment and prevention of blood clots all over the body. UFH has a broader anticoagulation scope and is typically used intravenously in hospital setting in connection with heart, vascular and plastic surgery, whereas LMWH is typically used subcutaneously (injection under the skin) both in hospitals and outpatient setting. UFH is a component of APAC and as such the closest comparator to APAC.
The cartoon and the table below show structural and functional comparison of APAC with the clinically used heparins.
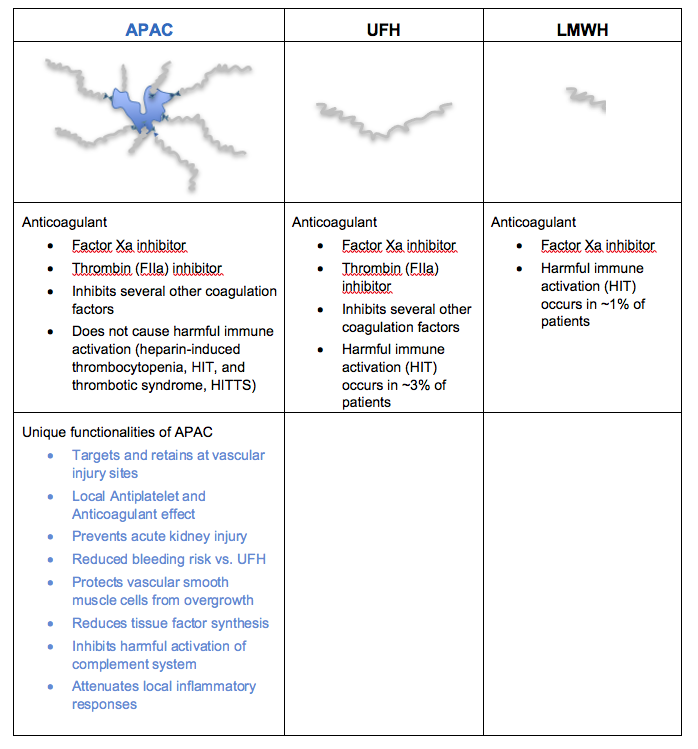
APAC retains the anticoagulant activity of the clinically used heparins, but APAC has several additional unique features as explained below. These unique features enable APAC to be used in indications for which there are no approved or appropriate therapies available and the medical need is high. A more comprehensive overview, as well as scientific publications, are provided separately.
APAC is a potent anticoagulant
- APAC carries the anticoagulant (AC) activity of the clinically used heparins. Based on preclinical studies APAC is in fact an even more potent anticoagulant than the clinically used heparins, enabling a smaller dosing.
- APAC’s anticoagulant activity is important as APAC can provide protection against the fibrin formation, where both platelets and coagulation factors are involved.
APAC is a potent antiplatelet agent
- APAC is a potent antiplatelet (AP) agent, strongly inhibiting collagen- and thrombin-induced platelet activation and aggregation. However, APAC does not prevent other platelet functions from initiating hemostasis, i.e., the adhesion of the platelets to the injury sites and the subsequent platelet activation, which is important to naturally repair the damage and stop the bleeding at the injury sites.
- APAC’s antiplatelet activity is important as APAC can prevent the initial platelet-driven vascular repair response from advancing to thrombus formation, especially at vascular injury sites in occluding arteries.
- Having both anticoagulant and antiplatelet activity is particularly useful and beneficial, and unique to APAC.
APAC targets the vascular injury site
- APAC finds its way to the vascular injury site and stays there for a prolonged time. If the vessel wall is intact (i.e. without injury), APAC will not bind.
- Vascular injury targeting is especially important in arteries where blood flow is high. In injured arteries APAC homes only to those sites which are vulnerable to blood clot formation (e.g. collagen under the endothelial layer exposed). Vascular injury targeting is a unique feature of APAC.
APAC targets the vascular injury site and prevents occlusion when administered systemically (intravenous IV) or locally
- APAC can be administered either locally or systemically, while being able to target and bind to the vascular injury sites. This unique ability enables the use of APAC in a wide range of potential target indications and provides flexibility to choose the best suited administration method for a given indication. For instance, a single, local administration of APAC is used in hemodialysis access failure indication, whereas intravenous (IV)-infusions are used in the COVID-19 indication.
APAC has a reduced bleeding risk vs. UFH
- When tested in a bleeding time model, APAC prolonged bleeding time less than UFH at the same heparin concentrations. Shorter bleeding time implies a reduced risk of bleeding, which is a potential serious adverse effect of all antithrombotic drugs (including heparins). The bleeding risk increases significantly when separate anticoagulant and antiplatelet drugs have to be combined to manage patients having acute arterial thrombosis. APAC provides both antithrombotic features in one product, but mainly locally and at a lower dose.
- APAC’s bleeding risk is further reduced by APAC’s targeting function. As APAC targets the arterial injury site and stays there for a prolonged time, the need for constant systemic anticoagulation and/or antiplatelet exposure may not be necessary.
APAC protects against acute kidney injury
- APAC protected against kidney injury in a rat acute kidney injury model. In contrast, UFH did not have any protective effect.
- Acute kidney injury is an organ damage, where blood supply is disrupted (ischemia), and with the help of a vascular intervention, the blood flow returns (reperfusion), to the oxygen deprived tissue (ischemia-reperfusion). Protection against ischemia-reperfusion injury is important as oxygen deprivation due to ischemia is a common feature in several APAC’s potential target indications (COVID-19, peripheral arterial disease/critical limb ischemia (CLI), and hemodialysis access failure).
- Furthermore, acute kidney injury (AKI) is a relatively common and often lethal complication in severely ill COVID-19 patients. Kidney damage (chronic kidney disease, CKD) is common in association with many vascular diseases. Diabetic patients often suffer from both CKD and CLI.
APAC has a number of other unique features
- APAC protects vascular smooth muscle cells
- APAC markedly protects vascular smooth cells (VSMC) from their overgrowth. APAC provides beneficial effects on VSMCs in in vitro (laboratory) studies by maintaining VSMCs physiological function of contraction rather than the overgrowth. At the tissue level APAC also attenuates inflammation caused by blood vessel occlusions and other inflammatory conditions.
- Effect on VSMC is important since after vascular injury (e.g. surgery to create an arterio-venous fistula for hemodialysis access) the harmful growth of VSMCs narrows the blood vessel lumen and increases the risk of occlusion. VSMCs play also an important role in the development of atherosclerosis (build-up of cholesterol plaque and inflammation in the artery walls).
- APAC reduces tissue factor synthesis
- Tissue factor is a protein present in deeper layers of vessel wall (underneath the endothelial cell surface in the interior of blood vessels) and leukocytes (white blood cells). Tissue factor plays a key role in the initiation of clot formation. Upon vascular injury APAC reduces the tissue factor level both at the injury sites and in blood circulation.
- Tissue factor overproduction is observed in many clinical conditions, including infection (e.g. COVID-19), cardiovascular disease (e.g. peripheral arterial disease / critical limb ischemia), cancer and diabetes. Tissue factor also promotes inflammation.
- APAC inhibits harmful activation of complement system
- APAC inhibits the unnecessary activation of the complement system, a part of the immune system. During inflammation, such as in severe COVID-19 / other infections, the inhibition of overactivation of the complement system is important to control its subsequent procoagulant actions.
Our business & market situation
Pharmaceutical industry
Aplagon is a pharmaceutical development company.
The pharmaceutical industry develops, produces, and markets drugs for use as medications. The size of the global pharmaceutical market was USD 1.25 trillion in 2019 [Statista]. The pharmaceutical industry is the largest investor worldwide in R&D [Schuhmacher et al. J Transl Med 2016]. 50% of the R&D pipelines of multinational pharmaceutical companies came from external sources (smaller R&D-focused companies like Aplagon and universities) already in 2013 [Schuhmacher et al. Drug Discov Today 2013]. The figure is today likely higher.
Development process for pharmaceuticals
Before a pharmaceutical product can be granted marketing approvals, it must go through a rigorous development process, which includes preclinical and clinical testing in compliance with international regulations. The testing is required to generate evidence on the safety and efficacy of the drug in the treatment of its target indication. It is often estimated that the development of a new drug takes 10–15 years.
The development process for pharmaceuticals consists typically of the following phases:
- Preclinical studies: Before clinical studies the candidate drug is extensively studied in preclinical setting. Such studies of the candidate drug involve both in vitro (test tube or cell culture) and in vivo (animal model) experiments to obtain preliminary efficacy, safety and pharmacokinetic (absorption, distribution, metabolism, and excretion) information.
- Phase 1 studies aim to study the safety, dosing, and key features of the candidate drug and are typically carried out in healthy volunteers. However, in some circumstances Phase 1 studies are carried out in patients, e.g. if surgery is involved (such as in hemodialysis access failure study with APAC).
- Phase 2 studies aim to assess the efficacy of the drug and gather further safety information.
- Phase 3 studies aim to provide a definitive assessment of the efficacy of the drug in comparison with the current best treatment. Phase 3 studies are typically randomised, controlled multi-center studies in a large number of patients. Market authorisation application can be submitted following successful Phase 3 studies.
Aplagon has carried out numerous preclinical in vitro studies using human blood and plasma, and in vivo studies in many animal models to test and show the function and efficacy of APAC. Aplagon has also carried out all toxicology studies in different animal species required by regulatory authorities to study dosing and to establish safety margins for the planned clinical studies for the selected indications. APAC is now ready to enter clinical studies in humans (Phase 1 & 2 studies).
Aplagon’s business model
Aplagon’s goal is to demonstrate preliminary efficacy and safety (clinical proof-of-concept) of APAC in at least two indications, COVID-19 and hemodialysis access failure, in the next 12-18 months and then find a commercialisation partner(s) for late-stage development and commercialisation of APAC outside India.
The partnering deal is expected to include significant milestone and royalty payments. Milestone payments alone for clinical stage pharmaceutical products typically exceed EUR 100 million.
The commercialisation deal for APAC would also provide an exit opportunity to the investors. The exit is likely to be through a trade sale to the commercialisation partner, license deal giving rights for APAC to the partner, or a merger with a listed company.
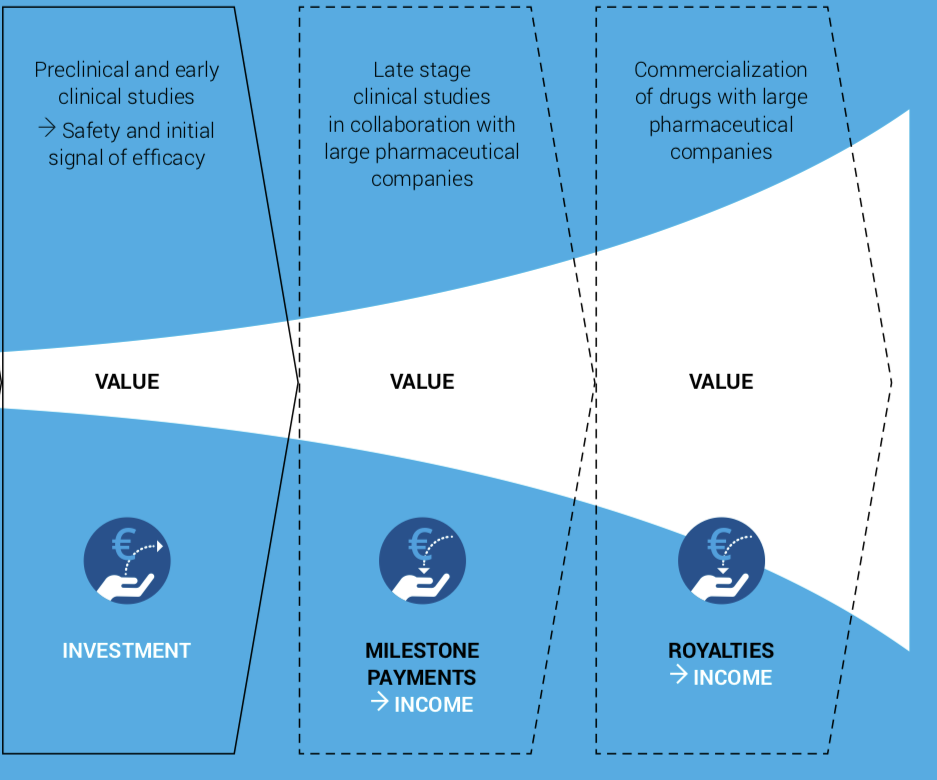
Source: a modified version of the chart in Herantis Pharma’s Annual Report 2019 .
Example of a partnering deal in a related indication:
- AM-Pharma, based in the Netherlands, develops a recombinant human alkaline phosphatase, a novel anti-inflammatory compound, for sepsis-associated acute kidney injury (SA-AKI). In May 2015, AM-Pharma entered an agreement with Pfizer. Under the terms of the agreement, Pfizer made an upfront payment of $87.5 million for a minority equity interest, and obtained an exclusive option to acquire the company, with additional potential payments of up to $512.5 million upon option exercise and potential launch of any product that may result from the agreement. AM-Pharma had at the time a Phase 2 clinical study ongoing in SA-AKI and had earlier completed a Phase 2 study with a bovine form of alkaline phosphatase.
Collaboration agreement with Cadila Pharmaceuticals
Aplagon and Cadila Pharmaceuticals (“Cadila”) entered in May 2017 into a development collaboration for APAC. Cadila is one of the largest private-held pharmaceutical companies in India. The collaboration covers the initial clinical proof-of-concept studies in hemodialysis access failure and COVID-19, and manufacturing of APAC for clinical studies. Aplagon has also an option for a Phase 2/3 clinical study in hemodialysis access failure in up to 360 patients. The clinical studies will be performed in India in a way that the data generated are fully acceptable also to the FDA (United Sates) and EMA (Europe). Cadila has the marketing rights for the Indian market whereas Aplagon retains the rights for all other markets. Aplagon’s responsibility is to make a commercialisation deal, once sufficient clinical data have been generated, for the late-stage clinical development and marketing and sales of APAC outside India. Cadila will pay Aplagon a modest royalty on Net Sales in India and Aplagon will pay Cadila a (reasonable) profit share on the ex-India commercialisation proceeds. In case Aplagon is sold, the profit share will be paid on the exit-proceeds instead of commercialisation proceeds.
The collaboration with Cadila provides Aplagon a highly cost-effective way of achieving preliminary efficacy and safety results in patients in two indications in parallel.
Initial target indications
Aplagon’s first clinical indications will be COVID-19 (systemic administration) and hemodialysis access failure (local administration). Aplagon is also planning to initiate a clinical program in advanced peripheral arterial disease / critical limb ischemia.
Aplagon expects APAC to be eventually used in a broad number of indications. The underlying causes – vascular injury, platelet activation and aggregation and activation of the coagulation system – play a key role in a wide range of diseases and disorders.
COVID-19
Description
COVID-19 is to a large extent a thrombo-inflammatory disease. Coronavirus disease 2019 (COVID-19) has caused an unprecedented health emergency, which was declared a pandemic by the World Health Organization (WHO) on 11 March 2020. The incidence, severity and mortality of COVID-19 is three times higher than that of ordinary influenza A. Patients hospitalized with COVID-19 are at high risk of developing venous and arterial thrombotic events (blood vessel occlusions) and microangiopathy (small vessel disease in lungs and other organs), leading to poorer outcomes and increased mortality especially in patient groups at high risk of thrombotic complications. [Zhou et al. The Lancet 2020; Cattaneo et al. Thromb Haemost].
Role of coagulation in COVID-19
Dysregulated blood coagulation drives disease progression and mortality in COVID-19.
The systemic activation of coagulation is reflected among others by the biomarkers fibrinogen and D-dimer. Fibrin is formed from fibrinogen in the coagulation process. D-dimer, a fibrin degradation product, appears in blood circulation, when blood clots are broken down by fibrinolysis.
A marked increase in D-dimer level is strongly associated with disease progression and mortality, as shown in the figure below.
D-dimer in COVID-19 patients
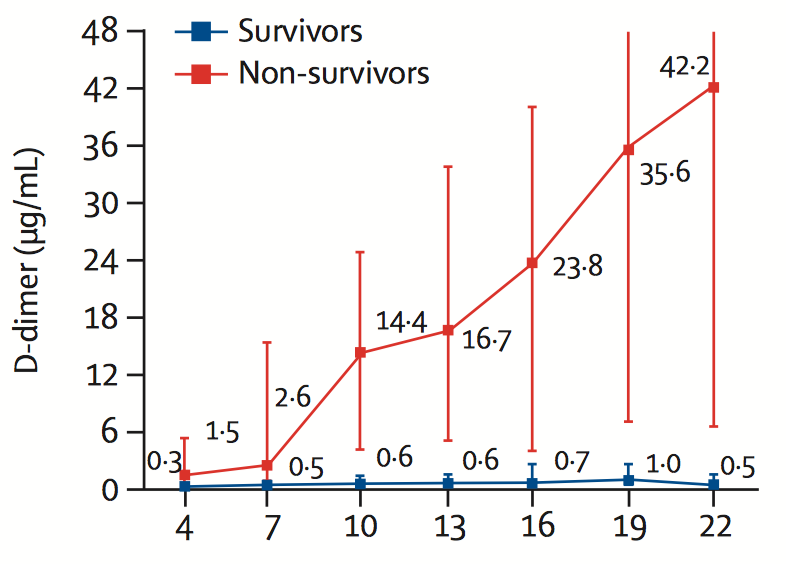
Zhou et al. The Lancet, 2020
The loss of oxygen and breathing problems as a result of lung inflammation and microvessel occlusions lead to the need for mechanical ventilation, which is typical for severe cases of COVID-19. Microvessel occlusions develop due to vascular damage caused by viral infection and severe inflammation. In autopsy reports, platelet-rich thrombi were detected in the lung, liver, kidney, and heart microcirculation. [Ackermann et al. New Eng J Med. 2020]. One third of severe COVID-19 patients have also venous thrombosis, which can cause life-threatening dislodging occlusions (embolisms) to lung arteries.
The picture to the right depicts a healthy (left side) and COVID-19 infected (right side) lung structures. Oxygen delivery through the infected lungs to blood microcirculation is further blocked due to fibrin formation and thrombosis. The outcome is acute respiratory distress syndrome (ARDS) needing critical care.
COVID-19 causes abnormal systemic coagulation escaping local regulation
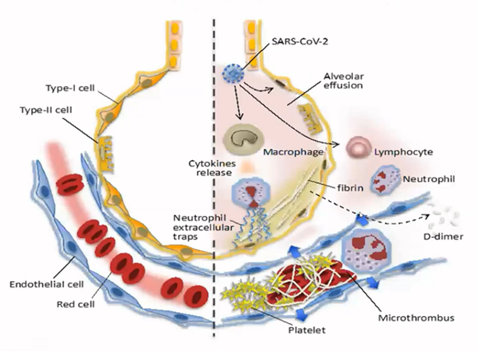
Iba T et al, 2020
Mortality of COVID-19 patients developing disseminated intravascular coagulopathy (DIC) / sepsis-induced coagulopathy (SIC), i.e., systemic dysregulated coagulation, can be up to 70%, vs 0.6% in non-DIC/SIC (Tang et al. JTH 2020). Severe COVID-19 often leads to multiorgan failure (lung, renal, heart, gut) due to microthrombosis (blood clots in small arteries). Acute kidney injury (AKI) carries extremely poor prognosis in patients suffering from COVID-19, with a high mortality of 76.5% (95%CI: 61.0–89.0) in a meta-analysis [Chen et al. Crit Care 2020].
APAC for COVID-19
APAC’s unique functionalities are a good fit with COVID-19 disease, as shown in the figure below.
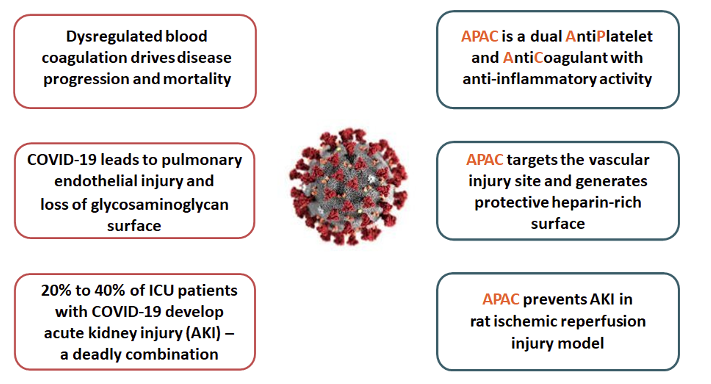
APAC is targeted as a treatment for hospitalised, high-risk COVID-19 patients. In the planned clinical study, APAC will be intravenously administered in patients who need additional oxygen but are not yet in mechanical ventilation and critical care, and who simultaneously have clear signs of enhanced coagulation activity (such as elevated D-dimer). The goal is to prevent the progression of the disease to a more severe stage.
COVID-19 – a stepping stone to other inflammatory coagulation disorders – a multi-billion euro market opportunity
Sepsis is defined as the body’s extreme, life-threatening reaction to infection or other severe inflammation, which can rapidly lead to vascular damage, organ failure, and death (Centers for Disease Control and Prevention, US). Sepsis-induced coagulation disorder, coagulopathy (SIC) – leading to systemic dysregulated coagulation – is a strong predictor of mortality in sepsis patients, with mortality rate over 30%. Microthrombosis in small arteries causes multiorgan failure and plays a key role in all forms of SIC, including COVID-19.
Sepsis is the leading cause of death in critically ill patients and the most expensive condition treated in hospitals. SIC is thus a multi-billion euro market opportunity.
Current treatment and competition
Treatment options for COVID-19 are limited. Certain antiviral drugs, corticosteroids and heparins have provided some benefits in addition to organ supportive care (e.g. hemodialysis). Treatment options for sepsis are quite similar, but in bacterial infections antibiotics are used.
There are many different therapies in development for COVID-19. However, none of these therapies has similar mechanisms of action to APAC, and most of them rather complement than compete with APAC (e.g. anti-virals and anti-inflammatory drugs).
Hemodialysis access failure
Description
Kidneys filter blood to remove wastes and extra fluids to produce urine and to maintain a healthy balance of water, salts and minerals, critical functions for the body. There are several conditions where kidneys lose most or all their function. Patients with end-stage renal disease (kidney failure) require hemodialysis, a life-saving treatment that cannot be conducted without a functioning vascular access. In hemodialysis the patient is regularly connected via a vascular access to a dialysis machine, which acts as a surrogate kidney. The patient’s blood is circulated through the dialysis machine to remove excess water, solutes and toxins. Dialysis treatment is usually administered three times a week and lasts a couple of hours.
The optimal choice for vascular access is an arteriovenous fistula (AVF). AVF is created by a vascular surgeon by connecting an artery and vein in the nondominant arm of the patient. Radio-cephalic AVF (radial artery sutured to cephalic vein) above the wrist is the recommended choice for hemodialysis vascular access. This site preserves the potential access sites further up in the arm for future AVF creation. AVF typically matures in four weeks and then dialysis therapy can be started. The figures below show how an AVF is created.
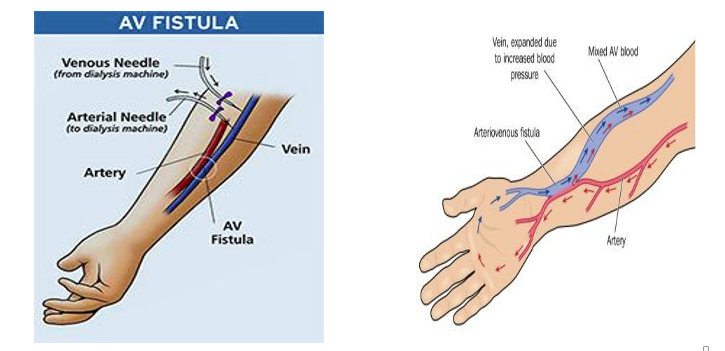
Creating and maintaining vascular access is challenging. Vascular access failure or dysfunction continues to be a leading cause for hospitalisation and morbidity in patients with advanced chronic kidney disease. In a recent Phase 3 clinical study in patients with a newly created radio-cephalic AVF, 47% of the AVFs did not mature properly and 69% lost their primary patency (failed) within the first year in the control group.
AVF failure is managed with surgical or interventional reconstruction procedures, but high rate of AVFs has to be abandoned and rebuilt. Morbidity, mortality and costs of care are high. FDA has designated hemodialysis vascular access failure as a fast-track indication, i.e. a serious or life-threatening condition for which there is no effective treatment.
Current treatment
There is currently no effective treatment for the prevention of AVF failure. Opening of AVF is usually attempted with balloon angioplasty (a dilation of the AVF site with a balloon) or a surgical revision. These procedures are costly, invasive, and associated with a number of complications, and often these interventions are unsuccessful or have to be repeated. For instance, according to the US guidelines the treatment goal for an AVF balloon angioplasty is to maintain AVF open for 6 months [National Kidney Foundation (US): Clinical Practice Guidelines and Recommendations for Vascular Access 2006].
Market opportunity
There is a high unmet need for an effective therapeutic treatment for prevention of hemodialysis vascular access failure. The cost of managing hemodialysis vascular access dysfunction in the U.S. is estimated to be USD 2.9 billion annually. In 2013-2014 there were approximately 429,000 hemodialysis patients in the U.S, 315,000 in Europe, 315,000 in Japan and more than 2 million worldwide, with an annual growth rate of 6-7%, mostly due to increasing incidence of diabetes. Approximately 130,000 AVFs are created in the U.S. each year. AVF patients on average require more than 1.5 procedures per year to maintain their AVF. Each operation typically costs between USD 5,000 and USD 15,000. An AVF costs on average more than USD 17,000 in the first year after surgical placement [Proteon Therapeutics 2017 10K filing]. According to equity analyst estimates a peak sales potential in radio-cephalic AVF maturation alone amounts to approximately USD 500 million worldwide.
Competition
An external blood vessel support device made of nitinol (VasQ) by Laminate Medical and sirolimus-eluting collagen implant by Vascular Therapies are in randomised clinical study phase for hemodialysis vascular access failure in the US. Both devices are placed around (outside) the AVF at the time of its creation. VasQ has received a CE-mark in Europe and is available for sale in Europe and Africa.
There are three segments within hemodialysis vascular access failure market: AVF maturation, AVF reinterventions and AVF maintenance. APAC is first targeted to the AVF maturation market but is expected to be applicable to all these market segments. VasQ device and the sirolimus-eluting collagen implant will only suit for the AVF maturation segment. APAC could also be used in combination with these devices in the AVF maturation segment, given APAC’s synergistic mechanism of action and as APAC is administered inside AVF, whereas the devices are placed outside the AVF. APAC could also be used as an anticoagulant in the weekly dialysis sessions.
Peripheral arterial disease / critical limb ischemia
Peripheral arterial disease (PAD) is caused by reduced blood flow (ischemia) to lower limbs due to atherothrombotic peripheral arteries. In PAD fatty inflammatory plaques build up on artery walls, triggering blood clot formation. PAD is associated with significant morbidity and mortality, as vascular atherosclerosis is typically wide-spread in PAD patients. PAD significantly increases the risk of heart attack and stroke, the leading causes of death due to arterial thrombosis, and lower limb amputation. Symptoms in the affected limb include claudication (leg pain when walking), reduced peripheral blood circulation, poorly healing wounds, acute or repetitive thrombosis, and tissue death. The treatment options are limited and include mainly life-style changes, the management of risk factors (hypertension, cholesterol) and long-term systemic antiplatelet/anticoagulation treatment, and revascularisation of the affected limb, and in unsuccessful cases leg amputation.
Dysregulated coagulation in the sick peripheral arteries supports the disease progression in PAD. Coagulation biomarkers, such as fibrinogen and D-dimer, correlate with the disease severity and functional outcomes. Coagulation disorder typically continues even after a successful revascularisation of the affected limb.
Critical limb ischemia (CLI) is the most advanced form of PAD (Rutherford scale 4-6), with highest mortality and amputation rates as shown in the figures below:
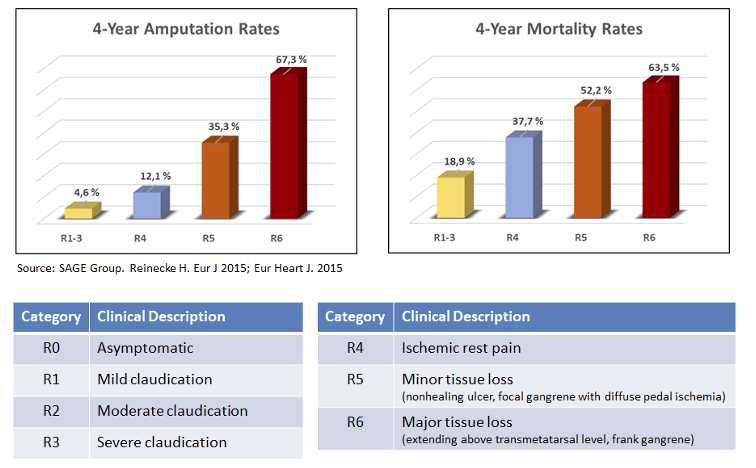
CLI alone is estimated to be a USD 12 billion market opportunity, with up to 7-8 million patients in the US and Europe alone.
Only a limited number of pharmaceutical therapies are in development for CLI and none with a similar mechanism of action to APAC. Most of the therapies in development are gene or cell-based therapies. These therapies aim to grow new vasculature in the affected limb in the subset of CLI-patients that are not candidates for revascularisation. The results to date have been inconsistent.
APAC targets the atherothrombotic blood vessels where it reduces the overactive platelets, coagulation and inflammatory processes. APAC can be used in connection with the revascularisations of the affected limb (surgery or balloon angioplasty and subsequent hospital stay) and as a maintenance therapy by intravenous infusions. Most of the therapies on the market or in development would likely be complementary rather than competitive to APAC.
Development plan
Aplagon’s main goal is to complete the Phase 1+2 study in COVID-19 and the Phase 1/2 clinical study in hemodialysis access failure (AVF maturation). Aplagon will also prepare for a Phase 2a clinical study in advanced peripheral arterial disease / critical limb ischemia and continue R&D work supporting the clinical development and partnering efforts. The studies will be performed in accordance with European (EMA) and US (FDA) regulations.
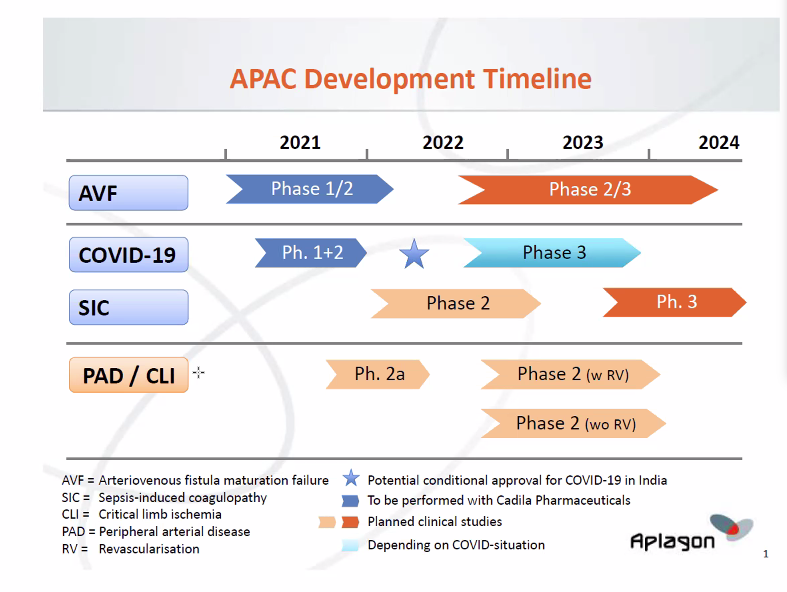
- Phase 1+2 clinical study in COVID-19
- The clinical trial application (CTA) for the COVID-19 study was submitted to DCGI, Indian regulatory authority, in early March 2021. Aplagon expects the study, given its focus on COVID-19, to be fast-tracked in the regulatory review process. The study is expected to start in Q2/2021 and be completed by end of 2021. The study is planned to be divided in two parts, Phase 1 and Phase 2.
- In the Phase 1 part, 10 healthy volunteers will receive four different dose levels of APAC. The primary objective of the Phase 1 part is to establish the safety and tolerability profile of ascending single dose and multiple dose IV (intravenous) administration of APAC in healthy subjects. Phase 1 will also determine the dose for the Phase 2.
- In the Phase 2 part, 60 COVID-19 patients will be randomised into two treatment arms. 30 patients will receive APAC and the standard of care (SoC, but without low-molecular weight heparin) and 30 patients will receive SoC. The primary objective of the study is to demonstrate the preliminary safety and efficacy of APAC in the study patients. Safety evaluation will include bleeding risk and potential other adverse effects. The efficacy endpoints include the change in disease severity based on the WHO ordinal scale, change in biomarkers (such as fibrinogen and D-dimer), duration of hospital stay and the need for invasive/mechanical ventilation. The patients will be followed up to 28 days.
- Phase 1/2 clinical study in hemodialysis access failure
- The clinical trial application (CTA) for the hemodialysis access failure study was submitted to DCGI, Indian regulatory authority, in Q2/2020. The review of CTA was delayed due to the COVID-19 lockdown in India but is now underway. The study is expected to start in Q2/2021 and take up to one year to complete. The study is expected to enrol 30 patients with end-stage renal disease, divided into three APAC dose groups of ten patients. In each group, eight patients will receive APAC and two will receive the vehicle. APAC will be administered locally in the arterio-venous fistula (AFV) during the surgical creation of a radio-cephalic (wrist-area) AVF. The primary endpoint will be safety. The secondary endpoints will include efficacy assessments related to the AVF-maturation (suitability for use in dialysis). The patients will be followed up for 4 weeks.
Other activities
- Aplagon is planning to continue among others the following activities:
- Preparations for a Phase 2a clinical study in advanced peripheral arterial disease (PAD) / critical limb ischemia (CLI) patients that show ongoing coagulation activity. The study will include a PET-imaging part with a radiolabeled APAC to determine the distribution and retention of APAC at vascular injury sites in these patients, and a short-term treatment part with an outcome and biomarker based follow up of the patients.
- R&D-work at the Company’s Biomedicum laboratory in Helsinki and with several international collaborators to support the clinical development and partnering activities.
- Formulation development work with Cadila Pharmaceuticals to optimise the formulation of APAC for commercial use.
- Discussions with potential commercialisation partners for APAC.
Patents
Aplagon has a strong IP protection for APAC through patents and data protection.
- Aplagon filed in August 2014 a patent application covering APAC and related molecular conjugates. The patent application entered national phase in February 2017 after receiving a favorable international patent examination in 2016. The patent has already been granted in Australia, China, Europe, Hong Kong, Japan, Mexico, Russia, Singapore and South Africa. The patent provides patent protection for APAC, without any patent term extensions, until August 2035. Aplagon is seeking to obtain patent coverage in all key territories worldwide.
- Aplagon filed in May 2020 a second patent application which is not yet public.
- The patents and patent applications are owned by Aplagon.
- In addition, Aplagon expects APAC to be entitled to 10 years of data exclusivity in Europe and 12 years in the U.S. after obtaining market authorisation.
Our team
Aplagon has a highly experienced and international team for the development and commercialisation of APAC. The operative team members have worked on the APAC development for years. Aplagon strives to work with highly experienced professionals while keeping the operations and cost base as flexible as possible, as such many of the team members are not directly employed by Aplagon.

Aki PrihtiCEO
Aki joined Aplagon as CEO in February 2015. Aki has more than twenty years of experience in life science growth companies, both in operational and board roles. Aki is one of the founders of Inveni Capital, a life sciences focused venture capital fund, and serves as a board member in Herantis Pharma Oyj, Medtentia International Ltd Oy (also as a part-time CFO on an interim basis), Onbone Oy and Aranda Pharma Oy. Aki has been involved in setting up and developing, as well as fundraising for, several life science companies.

Riitta LassilaCSO
Riitta is co-founder and CSO of Aplagon. Riitta is also Head of Coagulation Disorders at Helsinki University Hospital and Professor in Coagulation Medicine at Helsinki University. Riitta is an internist and has many years of experience across thrombosis and hemostasis research; she was previously a group leader at the Wihuri Research Institute in Helsinki. She is the author of over 300 peer-reviewed publications, review articles or textbook chapters. She has served as a steering committee member of EAHAD and EUHANET, a European hemophilia and allied disorders program. She sits on the advisory boards of several pharmaceutical companies. She is an elected member of the Finnish Academy of Science and Letters.

Annukka JouppilaResearch Scientist
Annukka is Biochemist and Research Scientist and has been involved in the development of APAC since the founding of Aplagon. Annukka is in charge of Aplagon’s laboratory at Biomedicum Helsinki and plays a key role in supporting third-party collaborators in manufacturing activities and preclinical studies.

Nick MeyersConsultant / Vice President, Product Development, Boyd Consultants
Nick is Vice President, Product Development at Boyd Consultants and works on a part-time consultancy basis for Aplagon. Nick is deeply involved in the APAC product development and chairs the biweekly project team meetings with Cadila Pharmaceuticals. Nick has over 20 years’ experience in pharmaceutical development, especially clinical studies and regulatory matters, having worked in senior roles among others at Alizyme Therapeutics Ltd and Phytopharm plc.

Bob HumphriesConsultant / Director, VisionRealisation
Bob is Director at VisionRealisation and works on a part-time consultancy basis for Aplagon. Bob has a leading role in clinical study related matters. Bob has formerly worked among others as Project Head for AstraZeneca’s antithrombotic drugs, ticagrelor and cangrelor. Both ticagrelor and cangrelor are currently on the market, with ticagrelor sales exceeding USD 1 billion.

Gijs van DedemConsultant / Advisor, member of Scientific Advisory Board
Gijs is a renowned expert in heparin chemistry and plays a key role in the manufacturing related activities. Gijs works on a part-time consultancy basis for Aplagon. Gijs is co-inventor of danaparoid (Orgaran®), a heparinoid antithrombotic drug developed by Organon and later acquired by Aspen Pharma. Gijs has served among others as Chief Biochemist of Organon, Professor of Analytical Biochemistry at Delft University of Technology, Kluyver, and is a member of among others The Royal Dutch Chemical Society and The Royal Dutch Biochemical Society.

Angela StokesConsultant / CEO, Sharp Regulatory Consulting Ltd
Angela is CEO / Director at Sharp Regulatory Consulting and works on a part-time consultancy basis for Aplagon. Angela has a leading role in regulatory related matters. Angela has over 30 years’ experience in regulatory matters having worked in senior roles in both leading CROs such as Syneos Health, where Angela served as Head of Global Regulatory Consulting, INC Research and Chiltern and in companies such as Eisai and Genzyme. Angela is also a board member of TOPRA, the organisation for professionals in regulatory affairs.

Simon CraigeConsultant / Director, Edge Toxicology Consulting Ltd
Simon is Director at EdgeToxicology Consulting and works on a part-time consultancy basis for Aplagon. Simon has a leading role in toxicology related matters. Simon has over 20 years’ experience in toxicology and preclinical development matters and he served previously as Manager, Non-Clinical – Regulatory Consulting at Syneos Health.

Sakari LassilaChairman of the Board
Sakari, co-founder of Aplagon, has over 30 years of experience in international commercial and investment banking, corporate finance and investment company management where he has held senior positions at Union Bank of Finland (now part of Nordea), Citibank, Alfred Berg and Carnegie. Sakari is now active as a Board member in private and listed companies and a non-profit foundation.

Harry HolthöferBoard member
Harry brings a wealth of international experience in translational research in diabetes, cardiovascular and kidney diseases. He served as Professor of Bioanalytical Sciences and Director and Chair of the Centre for BioAnalytical Sciences at Dublin City University from 2006-2016 and is currently Research Director at University of Helsinki, Finland, Adjunct Professor at University of Shenzhen, China and Visiting Professor at University of Hamburg, Germany. Harry is also co-founder and CEO of three start-up biotech companies.

Kai LindevallBoard member
Kai, co-founder of Aplagon and former President and CEO of Encorium Group, brings over twenty years of senior management and entrepreneurship experience in the life sciences sector gained at pharmaceutical, biotech and CRO companies. He was one of the founders of Remedium (a predecessor to Encorium) and Ipsat Therapies, and has served as Medical Advisor to Farmos, Medical Director to Rhône-Poulenc Rorer and as Associate Director of Research at Wihuri Research Institute in Helsinki, where he led his own research group involved in thrombosis research. Kai is the Chairman of the newly established company Siltana Ltd, developing new mobile solutions to improve drug safety and real world data access.

Steven MyintBoard member, Chairman of the Scientific Advisory Board
Steve is an advisor to the chairman of Singapore’s Agency for Science and Technology, amongst other governmental organisations. Previous roles included Executive Chairman of Green Signal Bio, one of India’s largest independent vaccine manufacturers, Global Medical Director of GlaxoSmithKline and Senior Vice-President for R&D/Chief Medical Officer at BTG International. He is actively involved in innovative biotech companies and co-founded Finland’s largest accelerator fund. Steve is a Fellow or Member of several UK societies, including the Institute of Knowledge Transfer, the Royal College of Physicians and the Royal Society of Medicine.

Scientific Advisory Board
Aplagon has a broad international scientific and clinical advisor network. Scientific advisory board members include Anders Albäck (Helsinki University Central Hospital, Finland), Douglas Cines (University of Pennsylvania, US), Adam Cuker (University of Pennsylvania, US), Alexander Cohen (Guy’s and St Thomas’ Hospital, King’s College, UK), Jonathan Himmelfarb (University of Washington, US) and Michael Kroll (MD Anderson Cancer Center, US).