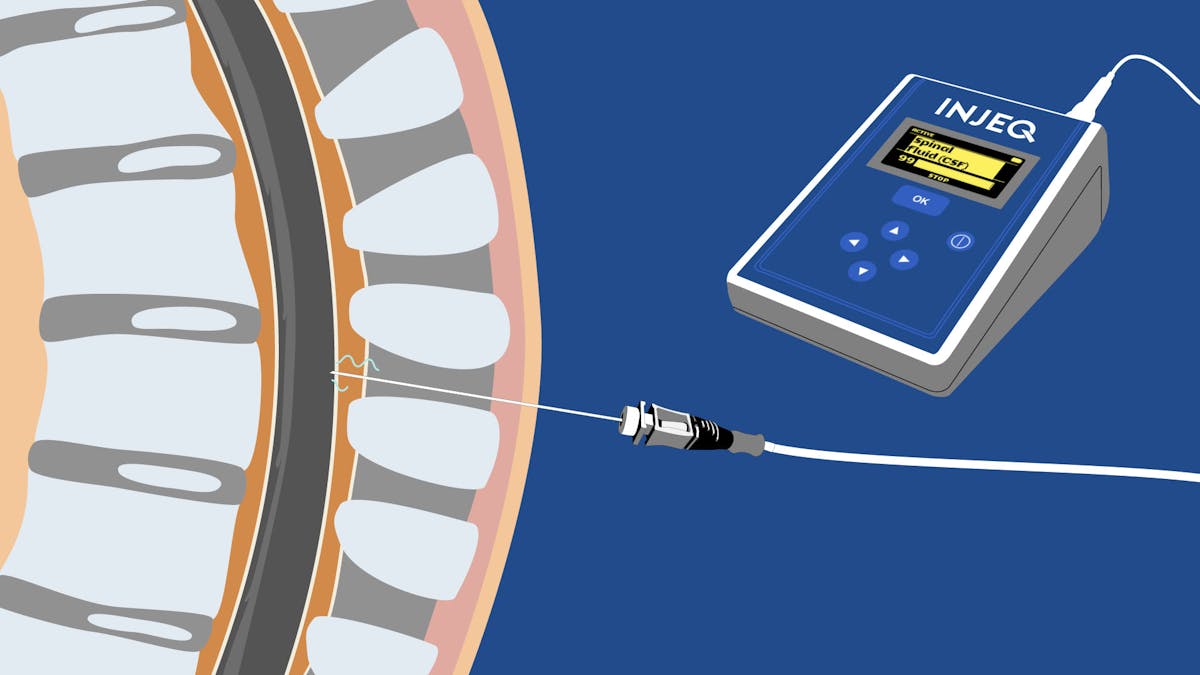
General Launch of the IQ -Tip® needle
The IQ-Tip®️ smart needle received CE approval under the Medical Device Regulation (MDR) in December 2021. Prior to the CE approval and marketing authorisation, the performance and safety of the bioimpedance smart needle has been studied in several different clinical trials. The scientific publications related to these studies can be found at www,injeq.com/professionals/publications/.
Studies
The applicability of bioimpedance for tissue recognition was first studied on adults at the Tampere University Hospital, Valkeakoski Regional Hospital and Koskis Hospital. The second study, focusing on lumbar punctures in new-borns and babies, was carried out in the neonatal wards of Tampere and Turku University Hospitals. The third study - leading to the CE approval - was conducted in the paediatric haematology departments of the University Hospitals in Tampere, Turku, and Helsinki on acute lumphoblastic leukemia (ALL) patients. The results of this study were presented in the spring 2021 at the Childhood Leukemia and Lymphoma Symposium under the title High first puncture success rate attained with a novel bioimpedance needle in pediatric hemato-oncologic lumbar punctures. Injeq's research engineer Sanna Halonen has also published on the applicability of bioimpedance measurement for liver biopsies and joint punctures, among others. Injeq's first post-market study is scheduled to start in the summer 2022.
Identified growth paths
Sales are initiated where the patient benefits are most obvious. Therefore, initial sales efforts will focus on paediatric oncology, such as ALL- and neonatal lumbar punctures.
Around 50 children are diagnosed with leukemia each year in Finland. By the same token, it can be estimated that in the Western world about 10 children per one million inhabitants are affected by this disease. Almost 90% of patients can be cured and of the remaining 10%, half die from the disease itself and the other half from the toxicity of the leukemia treatment. A failed lumbar puncture can be crucial for a leukemia patient which - in the worst case – can lead to death. A traumatic lumbar puncture with cancer cells entering the central nervous system (CNS) area – means additional doses of chemotherapy with increasing toxicity for the patient or possibly a relapse in the CNS area. Toxic treatment in the childhood can also lead to secondary cancers later in the adulthood. It is difficult to put a price on these complications, but the life-saving benefits of a successful puncture are a good justification for investing in a safe puncture and the IQ-Tip smart needle in primary cases. Patient safety also brings cost savings as the need for intensive care can be reduced.
There are about 3 cases of proven meningitis per 10,000 live births. However, this is an underestimate because about 30-50% of children with general infection (sepsis) in intensive care do not receive a lumbar puncture but are treated with antibiotics for sepsis. The lumbar puncture required for a diagnosis of meningitis may not be performed simply because of a challenging procedure. However, about one third of children with sepsis have negative blood culture results but still have meningitis. Infections of the central nervous system have a negative impact on the child's development and, in the worst cases, cause death. Delayed diagnosis can lead to life-long developmental disabilities. In an acute situation, incorrect or missing diagnosis can increase costs through prolonged stays in intensive care. The Injeq IQ-Tip® smart needle is expected to make lumbar punctures easier for even the smallest patients, lowering the threshold to perform this challenging procedure to ensure a correct diagnosis. With a smooth and safe lumbar puncture, the IQ-Tip smart needle can enable patients to have a better prognosis, save on intensive care costs and, in the long run, improve the quality of life of the patient while reducing costs to society.
Major European countries with higher population such as Germany, France and Italy offer significantly bigger market for the Injeq smart needle. Reseller agreements have already been signed in these countries, among others. To follow up the European market, Injeq plans to seek FDA approval and with it the US market between 2025 and 2026, when the market can be expected to grow significantly.
The first sales targets for Injeq resellers will be European children's hospitals, where Injeq products can be used to improve the success and safety of lumbar punctures. Children's hospitals operate both as part of university hospitals and maternity hospitals and as stand-alone units. Initial sales efforts will focus on leading university hospitals, which will also serve as reference clinics and promote the use of Injeq devices in areas such as paediatric ALL as part of the new European ALLTogether treatment protocol and other demanding lumbar punctures. Once a sufficient foothold in paediatric use has been achieved and the benefits of the technology have been demonstrated to clinicians, Injeq intends to use paediatric wards as references, highlighting the benefits and low risks of the technology to extend its use from paediatric to adult wards.
Business model
Injeq uses a dealer-operated sales channel. The resellers are selected based on their existing distribution field, currently mainly from resellers already active in the field of neonatology and paediatric oncology.
Injeq's business model is to sell through national resellers. The aim is to establish an EU-wide network of resellers to cover the main markets during the first two years of commercialisation. Injeq's management believes that this is a realistic objective and the first partnerships selected in the larger European countries show that the list of candidates is feasible and convincing. Discussions with leading Nordic university hospitals have also already taken place during the development phase of the smart needle. Discussions and previous clinical trials in several hospitals have shown that there is a very good basis for starting sales. Injeq has signed distribution agreements in Germany, Austria, Italy, France, Slovenia, Scandinavia, and Poland.
The sale of analysers as a one-off transaction will generate a faster revenue stream and its sale to the end customer will also trigger the sale of IQ-Tip® spinal needles and IQ-Tip® cables as disposable products. Depending on the target market and the financing models used by the Injeq reseller, part of the sales of analysers to end-customers can be leased or rented, thus lowering the acquisition threshold. In addition, Injeq will review the business and pricing models appropriate for the different target markets together with its resellers, so that pricing specifically for clinical opinion leaders and early-stage users will support faster market penetration. Injeq also plans to launch local studies and subsequent scientific publications in target markets to demonstrate the efficacy and usability of the IQ-Tip® smart needle at a local level. Injeq also has an obligation under the MRD to collect customer feedback. The feedback is collected from the doctors using the needle regularly.
There are countries like Slovenia where the distributor must give the device to the hospital for free and the revenue stream is entirely based on the sale of disposable products. In Germany, on the other hand, Injeq's strategy is to enter the market by contracting with local hospitals for trial distributions. Injeq has agreed a trial with two hospitals, for which the hospitals will receive the analyser and the first few needles free of charge and will then purchase additional needles themselves as needed. After a trial period of 4-6 months, the hospitals will make a final decision on the purchase of the device. The distributor redeems these devices from Injeq.
Lumbar punctures for adults
Focusing on a limited yet important segment, such as leukemia and neonatal lumbar punctures, as described above, is a sensible way to enter the market. In these indications, it is easier to justify the significantly higher price of a smart needle compared to a standard spinal needle. However, the number of lumbar punctures and spinal anaesthesia performed on adults is significantly higher than on children. For example, spinal fluid markers are increasingly being used to support the diagnosis of various neurological conditions such as Alzheimer's disease. The ageing population and the increasing prevalence of Alzheimer's disease are increasing the number of diagnostic lumbar punctures. New drugs for the treatment of neurological diseases have also emerged in recent years, which can be administered directly into the cerebrospinal fluid, where there is a need for certainty in dosing and where the cost of the injection may be very small compared to the cost of the drug. The company expects that over time the smart needle will become part of standard diagnostics and treatment practices, as the ease and safety offered by digital technology is highly valued. In addition, the IQ-Tip®️ smart needle can be used in the training of new healthcare professionals, as exemplified by a more than year-long collaboration with the Tampere-based Taitokeskus in medical education: every medical student is offered an opportunity to try out Injeq's IQ-Tip®️ smart needle during lumbar puncture exercises.
Expanding sales outside Europe
Investing in children's well-being is a global growth area. CE approval is a good basis for approval processes in many countries. New marketing authorisation applications and expansion of the sales network will be considered following EU approval. Product development towards FDA approval, as well as US regulatory actions and planning for the necessary clinical trials, will start during 2022. US market entry is targeted for 2025.