The information contained herein is not for publication or distribution, directly or indirectly, in or into the United States. These written materials do not constitute an offer of securities for sale in the United States. The securities have not been and will not be registered under the U.S. Securities Act of 1933, as amended, and are not being offered or sold in or into the United States.
The issue, exercise or sale of securities in the offering are subject to specific legal or regulatory restrictions in certain jurisdictions. The Company assumes no responsibility in the event there is a violation by any person of such restrictions.
Euroopan Aluekehitysrahasto (EAKR) has committed to subscribing 25% of the funding round up until €1,200,000. The first part of the commitment, €99,999, has been included in the investments and the progress bar of this funding round. The remaining part will be included in the investments and progress bar either when €900,000 has been received in investments from investors other than EAKR or when the funding round closes, whichever comes earlier.
The subscription for shares will be made in accordance with the terms of the funding round.
Investment information
- Type:
- Equity offering
- Invested:
- €2,000,367.00
- Equity offered:
- 3.34 – 14.75 %
- Price per share :
-
€3.00
min investment 400 shares
- Number of existing shares:
- 3,853,523
- Fully diluted shares:
- 3,973,523
- Pre-money valuation:
- €11,560,569.00
Our story
IQ-Tip®️ – Technology by doctors for doctors
Three experts in medicine and biomedical technology considered the problem of blind punctures in Tampere more than 10 years ago and decided to start developing a smart needle as the answer to the problem. The outcome is the IQ-Tip®️ spinal needle, which identifies cerebrospinal fluid as the first application.
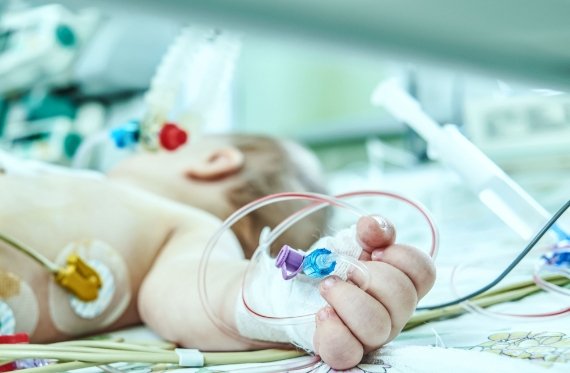
Injeq was founded when a group of technology experts discovered a solution to an unmet medical need. Riitta, Katja and Jari had a vision of a smart needle that has since advanced to being a finished product that is soon ready to be launched commercially. Upon securing marketing authorization in early 2021, the IQ-Tip® needle will first be used for demanding lumbar punctures (aka spinal tap) both in cancer units for punctures related to treating childhood leukemia and in neonatal intensive care units. The IQ-Tip® needle has demonstrated a good first-puncture success rate in clinical trials compared to the conventional needle studies in the literature, and it is expected to increase the opportunities for success in challenging spinal punctures on even the smallest of patients. Young leukemia patients and newborns are just two examples of the patients that benefit from the technology of Injeq’s smart needle.
Injeq IQ-Tip®️ smart needle – Aiming for safety in demanding punctures
Acute lymphoblastic leukemia (ALL) is the most common type of childhood cancer. A diagnostic lumbar puncture is performed at the onset of the disease to determine whether the cancer has spread to the central nervous system (CNS). Thereafter, lumbar punctures are needed for the follow-up of the disease and intrathecal administration of cancer drugs, both for prophylaxis and possible CNS disease treatment. The puncture to be performed on pediatric ALL patients is critical – especially if the disease has not yet spread to the central nervous system. A traumatic, suboptimal lumbar puncture may cause cancer cells to enter the spinal canal. This causes a risk of metastases occurring in the brain and central nervous system. In terms of the prognosis for leukemia, it is important to minimize all possibilities where this might happen; in other words, the spread of cancer to the CNS must be prevented. At the same time, the safety of lumbar punctures for the patient and the success of the puncture procedure must be maximized. According to Injeq IQ-LP-03 clinical study, which ended in autumn 2020, the first-puncture success rate using the IQ-Tip® needle was, compared to the literature, considerably higher than the using techniques with the conventional needle in a similar patient group.
Previously, pediatric ALL patients were treated in Finland according to the Nordic NOPHO – ALL 2008 treatment protocol. With this treatment program, more than 90% of ALL child patients survive, but children are still dying from the consequences of very toxic treatment. This has created a need to optimize the treatment: patients in the higher-risk group need stronger treatment than those in the lower-risk group. Therefore, the broader European ALLTogether study with 13 countries was established. In 2019, Finland joined the other Nordic countries in the study. The goal of the study’s research protocol is to improve the survival and quality of survival of patients with the disease.
Even though patients survive, some children with ALL still die of the disease after suffering a relapse. This is believed to be the result of under-treatment. At the same time, a significant fraction of young patients are “over-treated”, as a result of which they have an increased risk of treatment-related death, long-term side-effects and secondary cancers later in life. Mortality from ALL itself and from its treatment are similar. Treatment-related risks, such as lumbar punctures that cause hemorrhage, can be minimized by optimizing the puncture process using the IQ-Tip® needle. This study, independent of us, is perfectly timed to offer our distributors an optimal channel to deliver the IQ-Tip® needles to physicians who treat pediatric ALL patients. ALLTogether’s and Injeq’s shared intent is to provide small patients with higher quality treatment, with the aim of saving the lives of the “last” 10% of pediatric ALL patients.
The Injeq IQ-Tip® needle has also been evaluated in neonatal lumbar punctures in a clinical study which was completed in 2018. Newborn babies are vulnerable to meningitis in their first month of life. It is a very serious condition that left undiagnosed can quickly lead to death. A diagnostic lumbar puncture is, however, particularly challenging, as the procedure is usually performed without anesthesia. Injeq also wants to help make the first days of newborns easier. The smart needle is expected to reduce the need for unnecessary punctures, speed up diagnosis and thereby also reduce the number of days spent in the hospital and costs.
One case from our clinical study is a two-day-old baby on whom a CSF sample was unsuccessful even on the fourth attempt. Performed with the smart needle, it was successful on the very first attempt. The smart needle has been used successfully in our clinical study also for a puncture performed on a 1,4-kilogram premature baby.
The smart needle functions in a unique way
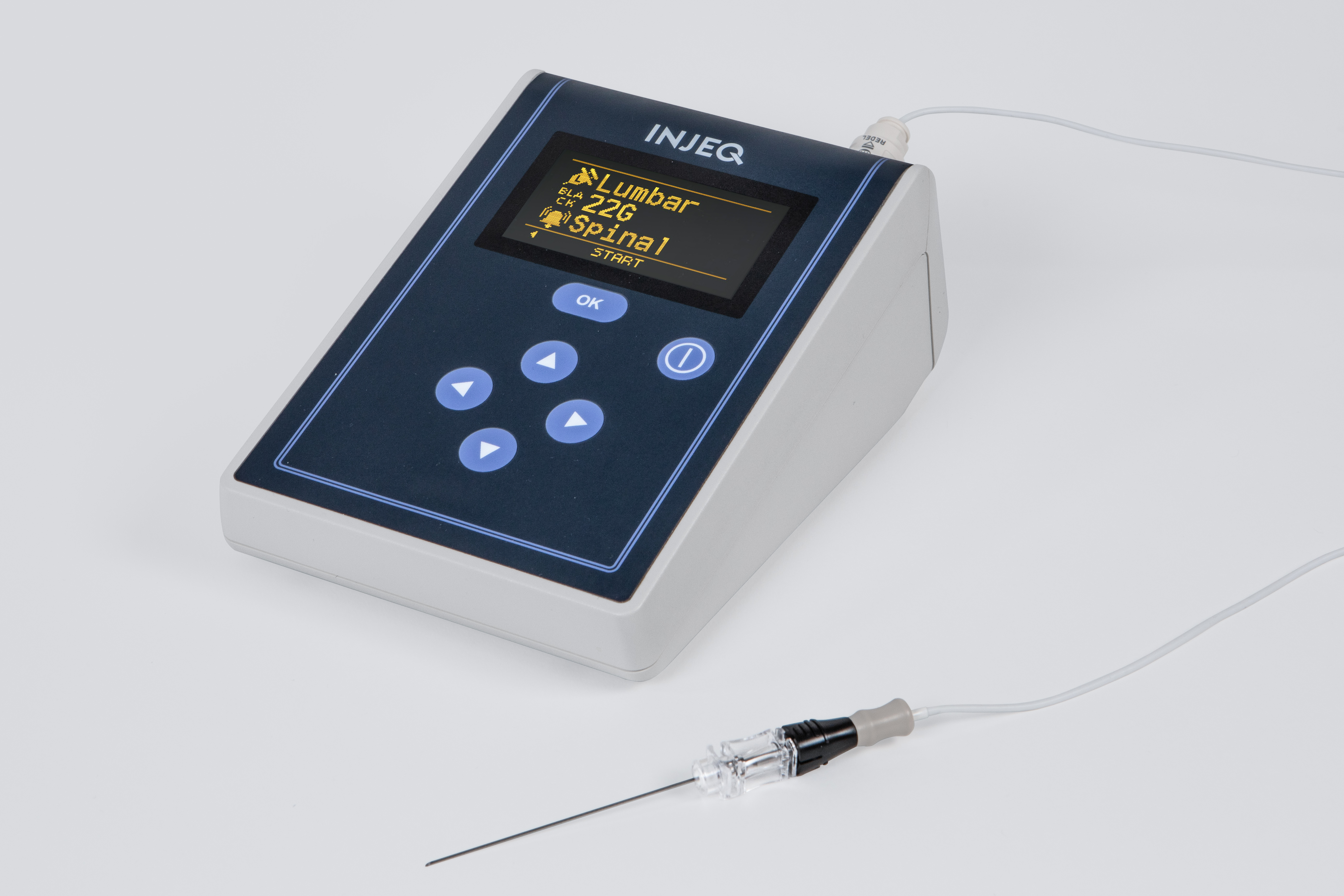
The Injeq IQ-Tip®️ needle works in a unique way: the needle’s body and the stylet inside it are used during the puncture as electrodes that measure the bioimpedance of the tissue that comes into contact with the tip of the needle. The Injeq IQ-Tip®️ analyzer does this 200 times per second with 15 different frequencies. This makes it possible conclude in real time when the needle tip reaches the target. During the lumbar puncture, the smart needle immediately informs the physician, on the analyzer’s screen and by means of an audio alert, when the tip of the needle comes into contact with cerebrospinal fluid; in other words, when the needle has reached the spinal canal.
We have studied CSF identification and the system’s safety in three clinical studies involving a total of 261 smart-needle-assisted lumbar punctures. More information on how the smart needle works and our previously published clinical studies is available on the company’s website, in the Publications section.
Wide-ranging applications offer extensive market potential
The IQ-Tip®️ needle will have numerous applications. In terms of lumbar punctures, the most demanding procedures are punctures on newborns and pediatric ALL patients. In this target group, the benefits of the smart needle and the need for it are so obvious that these punctures are the first commercial applications for the smart needle. Other potential applications – some requiring new clinical trials and product versions – are, for example, cancer medication injections, cancer samples from internal organs, myelography, Alzheimer’s diagnosis, joint punctures and blood vessel punctures. These clinical applications offer extensive future growth opportunities. Possibilities that can be considered in the long term are special application areas offered by the field conditions of first aid and military medicine, where the needle can help deliver immediate help the right way even in challenging circumstances. Every application area in and of itself represents a considerable market, and the overall market potential is therefore very broad.
Injeq’s smart needle aims to become part of standard treatment
There is no product in the market like the Injeq IQ-Tip®️ needle, and the company is not aware of potential competitors that are close to entering the market. Similar attempts to make injections easier has been developed by various research groups, for example, using techniques based on pressure sensing or light scattering. These techniques and their applicability, however, have shortcomings and more precise information on their market entry is lacking. The company therefore has an excellent opportunity to establish a position as the new standard in medical procedures involving demanding punctures. In clinical patient studies, the Injeq IQ-Tip®️ needle has proved to be effective and safe. The product will be ready for commercial launch as soon as the clinical evaluation of the Injeq IQ-Tip®️ needle has been approved by the notified body and the product has been successfully registered in compliance with the more stringent requirements of the Medical Device Regulation (MDR). The company expects this to take place in early 2021.
Why invest in Injeq?
After the clinical trials the company has a unique product in its hands – smart IQ-Tip®️ needle – ready for commercialization to the growing healthcare markets, enabling improved success and safety of challenging lumbar punctures.
- The company expects certification of the quality system and the products to be completed during January-February 2021, enabling start of sales in Europe thereafter.
- Clinical trials have been completed with good results. The company is commercializing the first products in accordance with the high requirements of the latest Medical Device Regulation.
- Regulatory approval and so-called marketing authorization process with all the audits is already in the final stages by the notified body TÜV SÜD.
- The company has a competent and experienced staff that, together with the stakeholders, is able to commercialize demanding medical technology products starting from the smart needle as the company's first commercial product.
- Injeq smart needle aims to turn challenging lumbar punctures, and later on other demanding punctures, even safer for patients.
- The company aims to help in the treatment of children with leukemia and to minimize the harmful effects of traumatic lumbar puncture in a growing number of patients.
- Customer needs and user feedback about new possible application areas received during the trials offer diverse product and growth opportunities to utilize the company's industry, process and commercial expertise.
- The company has secured its intellectual property rights by protecting the applied technology with patents.
- As an investment target, we combine the pursuit of financial performance with ethical goals. As a company, Injeq primarily seeks to develop new methods and treatment practices for the benefit of the patient - which is the key for a successful and sustainable business within the demanding medical technology sector.
Since the last funding round
A lot has transpired since the last funding round with Invesdor in summer 2019, most importantly that the MDR-compliant product approval and marketing authorization process for the IQ-Tip®️ needle is under way with the notified body TÜV SÜD.
In 2019, we found suitable new business premises with production areas and a cleanroom suitable for the company’s operations. Besides product approval measures, we have also recruited excellent new professionals, built our future distribution and sales network and maintained our patent portfolio. In 2020, the company was granted three patents, two in the U.S., and the one related to a biopsy needle also in Europe. One of the U.S. patents expands the invention protection applied to our first product, which is in the commercialization phase, beyond Europe.
The new MDR regulation on medical devices has significantly hampered the availability of notified bodies required for device manufacturers’ product approval procedure, especially for newcomers in the field. There were only slightly more than ten confirmed notified bodies under the new MDR regulation in all of Europe in spring of 2020 when our registration process began, compared to roughly 50 previously. In this market situation the notified bodies, which continue the certification of medical devices, can choose customers carefully. We can be pleased having been selected as a customer by the well-respected body in question, TÜV SÜD.
At the same time, we have focused on getting our processes and our quality management system ready and in line with the requirements in order to be granted market authorization. All in all, this is a very time-consuming process – particularly with respect to our smart needle, which is a Class III device. In order to fulfil all the requirements set for the product and production, while minimizing the risks over its entire life cycle, we have been required to carry out a large number of product approval tests and, among other things, process validations. The suitability of each stage of the production process for routine production has been demonstrated through a multi-part validation procedure. Audit and inspection of validations by a Notified Body is part of the product approval process.
The key target this year has been, in addition to certifying the quality management system, completing the clinical investigation, and the CE approval process that is conducted based on it, in line with the new Medical Device Regulation. Our clinical investigation that was launched in November 2019 proceeded well until its conclusion, despite the exceptional circumstances. After reaching the planned number of patients and procedures, and once the month-long follow-up period for the last patients ended, the study was completed in September 2020. The results of the study, which involved 50 pediatric ALL patients and 152 procedures, were then analyzed together with researchers. The first-puncture success rate was significantly better than the acceptable limit that we had initially set for the study based on the literature. Another considerable benefit is related to the quality of the cerebrospinal fluid sample, with the study samples showing a much higher rate of blood-free samples than the reference samples. In addition, the most common unwanted after-effect of a lumbar puncture, i.e. post lumbar puncture headache, occurred less frequently in our study data than what was reported in a meta-analysis and review article published in the highly respected The Lancet journal in 2018. The detailed results will be published in an international medical publication aimed at professionals in the field.
The audit by our notified body, TÜV SÜD, is ongoing as part of the certification process to obtain marketing authorization for our products. The first audit of our quality management system has proceeded smoothly, despite autumn’s exceptional circumstances. TÜV SÜD’s experts recently visited us to audit our processes and our cleanroom, deeming them appropriate. We have already addressed the observations brought up thus far in the audit. An inspection and evaluation of the technical documentation for the Class III product, i.e. the needle, is currently under way as the final part of the audit. The registration process is therefore, for our part, in the final stretch. We will carry out the most important development measures that are required already in November and December, and we expect to have authorization to market the products early 2021, depending on the processes of the notified body and the EU, which will still take a bit of time after the completion of the audit.

Our business & market situation
The product and demand
The company’s first product is the IQ-Tip®️ spinal needle, which can be used to take a cerebrospinal fluid (CSF) sample and to administer intrathecal drugs into the CSF, for example, as part of leukemia treatment. These lumbar punctures are especially demanding for small children, and Nordic pediatric university hospitals in particular have expressed great interest in securing the product for their use. In the case of the most common childhood cancer, i.e. ALL-type leukemia, a lumbar puncture can typically be performed on a single patient approximately up to 20 times. The risks involved in the procedure could lead to tissue damage, increase pain and suffering and complicate treatment. According to a comprehensive Canadian study, hemorrhage into the spinal space from the effects of a traumatic lumbar puncture was associated with lower survival rates (Shaikh et al. 2014).
Besides cancer treatment, another typical reason for a lumbar puncture is suspected meningitis, which requires a high-quality CSF sample to diagnose. Left untreated or undiagnosed due to a poor sample, meningitis is always life-threatening. With newborns, obtaining such a sample is associated with problems that the Injeq IQ-Tip®️ needle is expected to resolve. According to studies, roughly 50% of punctures on newborns fail or cause tissue damage (Olowoyeye, 2019). Unsuccessful punctures, poor samples and the subsequent delays they cause in the patient’s treatment, not to mention possible return visits by the patient, cause not only pain and suffering but also additional costs as the child’s intensive care is unnecessarily prolonged.
We expect our product to also provide benefits in procedures performed on adults. Our goal is that once the smart needle has established its position in pediatric lumbar punctures, the method will then be used first for challenging lumbar punctures on adults and from there extended to everyday use covering all patient groups.
Our business model
The market potential in European pediatric hospitals is estimated at more than 50 million euros per year, based on the number of patients, procedures and hospitals, and estimated sales prices. The greatest need for the product is in the treatment of childhood leukemia and diagnostics and intensive care for newborns. These specialized fields account for around 60% of the medical procedures carried out on children. Our sales strategy is to cover each pediatric hospital’s total need for demanding lumbar punctures within a reasonable transition period to ensure consistent operating methods.
The potential available market (PAM) for Injeq’s smart needle can be considered to cover the global consumption of spinal needles used for all possible punctures. This naturally would amount to several billions of euros. The total available market (TAM) is substantially smaller. Since we are targeting the global market only in applications where IQ-Tip®️ technology brings a significant added benefit to the existing procedure compared to current products, the market shrinks further, with the serviceable available market (SAM) expected to be in the region of one billion euros, depending on the technology’s future price erosion.
Both the sales approach and pricing model will vary according to the target market and partly by distribution partner. The product system comprises a tissue analyzer, single-use needle and a cable that runs between them. The one-off sales of tissue analyzers will generate a faster income flow, and when the analyzers are sold to end customers, this will launch sales of smart needles as disposable items. Depending on the target market and the financing models adopted by our distributors, the analyzers will, however, often be placed to customers through lease financing or a leasing contract, which will lower the procurement threshold. Some may naturally be placed directly, in which case the procurement is limited to products sold as consumables. We will look at business and pricing models adapted to different target markets together with our distributors, also in terms of ensuring that the pricing, especially for opinion leaders and early users, supports faster market penetration. We aim to initiate local studies and subsequent publications in the target markets demonstrating the product’s efficacy and applicability also on the local level.
Sales, manufacturing and distribution
Sales and marketing of the products in Europe require MDR-compliant product approval and certification (CE marking) by the notified body, and for our smart needle also by the EU. The company expects to conclude its required registration-phase tasks at the end of 2020. With the notified body’s and the EU’s processes, we expect the final registration to take place in early 2021.
Our first sales target is children’s hospitals in Europe, where our products will be used to improve the safety and success rate of lumbar punctures. Children’s hospitals operate in connection with university and maternity hospitals, and also as separate units. Sales efforts will initially focus particularly on leading university hospitals that will also serve as references and will promote the use of Injeq’s devices as part of the new European ALLTogether treatment protocol, especially for ALL-type leukemia and other demanding lumbar punctures.
Injeq owns the main rights to its products and acts as the manufacturer of the products, overseeing their safety. The needle and cable are manufactured in the company’s production facilities in Tampere from components procured from several different manufacturers. In summer 2019, the company moved to new premises in the area of Tampere University Hospital. These suitable premises enable increased production as sales grow. The products are sterilized by a European cooperation partner. The analyzers are made on a subcontracting basis by a Finnish contract manufacturer.
Sales are implemented with local distributors, primarily with regional companies who already have established local operations with children’s hospitals. Injeq has identified approximately 450 potential operators/distributors around the world, among them several European distributors who have been selected for more detailed negotiations. Preliminary distribution agreements have been concluded in five European countries, and discussions with distributors in several other countries have been initiated. The aim is to establish an EU-wide distributor network in the course of 2021. This is a realistic target, and the first partnerships selected in the larger European countries prove that the list of candidates is feasible and compelling. Collaboration with Nordic university hospitals will continue. Negotiations have been under way with leading Nordic university hospitals throughout the smart needle’s history. Discussions and previous clinical trials in several hospitals have proved that when it comes to launching sales, we are on a solid ground.
Competitive situation
Spinal needles for lumbar punctures are widely used around the world. Every year, some 100 million lumbar punctures are performed, and the procedure dates back more than a century. The current practice is both a competition factor and an opportunity. Opportunity stems from the fact that the current practice involves problems and risks that the Injeq IQ-Tip®️ needle is able to minimize, while a competitive situation arises if the treating physicians believe the existing method is sufficiently effective and affordable. However, many pediatricians are unsatisfied with the current solutions, since lumbar punctures are demanding, particularly when it comes to children, and there are more risks associated with using a conventional needle with this patient group. That is why the company has focused its resources first on the needs of children, a route which is then expected to establish the smart needle as the new standard in procedures performed on adults, too.
The smart spinal needle does not yet have its own market segment, because the products are not yet commercially available. Injeq Oy will thus be the technology and industry leader when the product is launched. Established needle manufacturers are familiar with the company and are following its progress. And with good reason, as the company’s vision is to revolutionize the existing treatment practice. For some needle manufacturers, this could mean weakened sales. As we achieve our goals, the use of conventional needles for the applications in question will decrease – one hospital unit at a time. However, there is an opportunity on the global markets to partner with conventional needle manufacturers and take advantage of their logistics and sales & distribution channels.
The problems related to lumbar punctures are widely known, and attempts have been made to develop competing solutions based on, for instance, measuring pressure or light scattering at the tip of the needle. However, the use of pressure measurement, i.e. the manometer-based technique, has its limitations, and light-based solutions are not yet in the commercialization phase. It is safe to say that there are no competitors in the market who can match our product, and their product development work and commercialization lag behind Injeq’s.
Quality Management System and approval of a medical device
The manufacturing of medical devices is regulated by the authorities. The authorities have outsourced the control of regulated activities to Notified Bodies approved by the authorities. According to the regulations, Injeq must have a quality management system, approved by the Notified Body, covering the minimum requirements set out in the regulations. The requirements are more demanding than the normal quality management system. In order to minimize the risk to the patient to an acceptable level, the measures defined in the quality management system related to e.g. product development, manufacturing, product testing, product’s clinical investigations, marketing and post-market follow-up of a product to be commercialized must be approved when the product is launched to the market.
We moved our operations to production-scale premises in summer 2019. The necessary quality management system, which is currently being certified, has been in use for a longer time. In the company’s estimation, clinical evidence based on the final studies is expected to be approved by the notified body early 2021. In addition, the company is in the final stretch of completing all testing and audits in compliance with the EU’s regulatory requirements. After obtaining an EU marketing authorization, the company intends to apply for marketing authorizations in other geographical areas, primarily in areas where the CE marking is approved as is or with minor adjustments, as well as initiate preparations for other larger market areas (such as the U.S., which requires its own FDA approval - The Food and Drug Administration).
Medical Device Regulation and regulatory requirements
Although the development work and commercialization of new medical devices in compliance with regulatory requirements is not always straightforward, gaining access to the market often establishes a firm position for the product. An essential aspect of commercialization is the regulatory approval required for medical devices. New requirements were set for the highly regulated industry in 2017. The EU’s regulatory framework changed from a Medical Device Directive (MDD) to a Medical Device Regulation (MDR) and will apply directly to all member states’ legislation. The transition period between the directive and the regulation for medical devices ends in May 2021. The practices under the more stringent MDR are thus not yet fully established. The new MDR has also made the certification process more demanding. Brexit also has not helped the situation, as roughly 30% of the notified bodies’ resources were in the U.K. All of this creates waiting lines for the customers of European notified bodies. With regard to the requirements of the MDR, Injeq has started preparations with notified body TÜV SÜD already in 2018.

A sustainable competitive edge
The company’s products cannot be easily copied by competitors. Copying or replacing either the needle or the analyzer is not a straightforward process, and to protect them, the company has either been issued or has applied for patents covering four different technologies. The company’s business is protected by the patents to a certain extent and, in addition to these patents, the data generated in studies and clinical trials, which cannot be patented or copied, provide a sustainable competitive advantage. Competitors must also conduct similar clinical trials, which gives Injeq a clear head start in the market.
The company’s algorithms are confidential, and measurement data is gathered when the products are used. This enables the continuous development of the products and the optimization of the algorithms. The company’s objective is for the Injeq IQ-Tip®️ needle to eventually become the best treatment practice.
Growth paths
The company’s first market segment will be lumbar punctures performed on children in European hospitals, particularly in connection with the treatment of acute llymphocytic leukemia (ALL). Success in this market is a key goal for the company. The aims of the four-year-long multinational ALLTogether study launched in 2019 are very much aligned with the benefits brought by Injeq’s smart needle. Our distributors in Europe are being offered the opportunity to join the efforts to improve the treatment and prognosis of pediatric ALL, as driven by this current European study.
Proactive measures to create distributor and sales channels have been initiated in Europe. Once the CE marking has been granted, we intend to expand the company’s operations to countries that accept the certification. This will give Injeq major market potential in the early phase of commercialization. Once the first foothold is established, there will be several more paths to growth. Investments there will be considered on a case-by-case basis. The main growth paths that have been identified include, but are not limited to:
- Lumbar punctures on adults. Focusing on a limited and at the same time important segment, such as pediatric treatment, is the sensible way to enter the market. However, lumbar punctures and spinal anesthesia are performed on adults considerably more than they are on children. For example, cerebrospinal fluid biomarkers are increasingly being used to support a diagnosis of Alzheimer’s disease (Paterson et al. 2018). The aging population and increase in the incidence of Alzheimer’s disease are increasing the number of diagnostic lumbar punctures. Once the product has gained a strong enough foothold in pediatric use and the benefits of the technology have been demonstrated to clinics, the intention is to use the pediatric clinics as references, highlighting the benefits and low risks of the technology in order to expand its use from pediatric units to other units. After overcoming initial obstacles and gaining approval for the IQ-Tip®️ system, this transition will be relatively easy. In the company’s view, new devices or other local distributors will then not be needed, nor will clinics need to learn new skills. The company expects that the smart needle will eventually be part of regular diagnostic and treatment procedures, as the easy-to-use and safe digital technology is greatly appreciated. The smart needle can furthermore be used to help train new healthcare professionals. This customer need has become evident in connection with the Injeq’s clinical investigation.
- Expanding sales to other global markets. Focusing on children’s well-being is a growth area also outside Europe and the U.S. For many countries, European product approval serves as a good foundation for their respective approval processes. New marketing authorizations and the expansion of sales networks will be considered once EU approval has been gained.
- Initiating preparations for FDA approval and expanding sales to North America. Preparations to initiate the FDA approval process, along with new patient studies, can begin once EU approval (CE marking) has been granted to the IQ-Tip®️ product and the product is in the market. As the world’s largest medical product market, the U.S. offers significant opportunities.
- New product areas. Besides lumbar punctures, IQ-Tip®️ technology can also be used for numerous other demanding procedures, such as joint and blood vessel punctures, biopsies, as well as in veterinarian medicine and first aid, to name a few. The company will study the various opportunities in these application areas after the smart needle’s first application has entered the market.
Our team
The commercial importance of health technology has grown in Finland year on year. Currently the sector is one of the fastest-growing high-tech export sectors in Finland (Healthtech Finland export statistics). Injeq operates in Tampere, where the cooperation with Tampere University Hospital and Tampere University has built up a successful ecosystem between research and businesses over the past few decades. Tampere is by no means lacking in experts, but there is competition for the best resources.
On this foundation, Injeq has been able to assemble a competent and experienced team. The team consists of experts with experience in a wide range of fields including medical and clinical research, regulatory requirements, signal processing and various areas of sales, production, quality assurance, and international corporate management. The company has an active Board of Directors that includes the original experts and developers of the innovation. The Board is further supplemented by outside advisors who want to facilitate IQ-Tip® products to become successful.

Ilari AntilaChief Executive Officer (CEO)
M.Sc. in Industrial Engineering and Management, 30 years of experience in international sales, marketing and management positions in several European countries with global health technology market leaders and in management of SMEs and startups. Together with international teams of high-tech experts, commercialized dozens of systems and hundreds of products for the global health technology market.

Seppo LautamäkiChief Commercial Officer (CCO)
Senior sales professional with 30+ years of experience in international technology sales. Responsible for developing our sales channel and commercial partnerships.

Maarit ForsténClinical Application Specialist
M.Sc. in Biomedical Engineering; Registered Anesthetist Nurse. 2020 Certified ISO 13485:2016 Lead Auditor. Clinical specialist and product specialist, responsible for sales support and distributor training. Years of experience in international distributor cooperation on the European market, organizing sales and training events and European research.

Timo ElomaaChief Operating Officer (COO)
M.Sc. (Industrial Engineering and Management) with over 20 years of experience in innovative high-technology products. Responsible for R&D and a wide range of general management tasks.

Harri SievänenChief Scientific Officer (CSO)
D.Sc. and Adjunct Professor (Biomed. Eng.). Over 30 years of experience in clinical science and experimental studies and their application to medical devices. More than 320 peer-reviewed scientific publications. Responsible for clinical investigations.

Juho KariResearch Engineer
M.Sc. (Medical Physics, Biomedical Engineering) Scientific expert in clinical investigations and clinical evaluation, product verification and validation testing and data analysis.

Sanna HalonenResearch Engineer
M.Sc. (biomeasurements), PhD student in biomedical engineering. Scientific expert in clinical investigations and data analysis.

Jussi SeitsonenChief Quality Officer (CQO)
M.Sc., majoring in microbiology. 20+ years of experience in quality systems in microbiology and quality assurance in the development and manufacturing of pharmaceuticals and medical technology products.

Petri AhonenChief Manufacturing Officer (CMO)
M.Sc. (Production Technology) with 20+ years of industrial experience. Responsible for production, including development of production processes, facilities and devices.

Katja SillanpääProduction Engineer
Production maintenance, monitoring and testing. Cleanroom specialist.

Manuela VillarrealProduction Specialist
Product manufacturing, production process development and training.

Eeva SelänpääProduction Specialist
Product manufacturing, production process development and training.
Terttu KatajaProduction Specialist
Product manufacturing. Years of experience in manufacturing medical devices.

Tommi RasilaChairman of the Board
Board professional with over 30 years of experience of entrepreneurship. D.Sc. (Tech.) with a dissertation on growth company processes. As the chairman, his role has included leading the strategic planning, business development and financing of the company.

Riitta Seppänen-KaijansinkkoCofounder and Deputy Chairman of the Board
Prof, MD, DDS, PhD, FEBOMS (Fellow of European Board of Oral and Maxillofacial Surgery), PhD Tech (h.c.), Specialist in Oral and Maxillofacial Surgery (med & dent). Over 30 years of experience in clinical work, research and entrepreneurship.

Jari HyttinenCofounder and Member of the Board
PhD (Technology) and professor of medical technology. 30 years of experience in research and education on medical devices and medical technology, and over 15 years of management experience in research and international organizations.

Katja PaassiltaCofounder and Member of the Board
DDS and M.Sc. (Tech.). Specialist in Prosthodontics. Innovator of the original smart needle.

Tommi MajausMember of the Board
M.Sc. (Tech.). CEO of medical device manufacturer Atrotech.

Kari JääskeläinenMember of the Board
BBA & MBA, CBM. Health technology and management professional.

Jan LindgrenMember of the Board
MD, PhD (Medicine and Surgery). Theoretical and practical knowledge in medical practice combined with relevant networks.

Leena LindgrenAdvisor
Prof. em. Anesthesia. Vast theoretical knowledge and practical experience in needles and blind punctures.

Petri PommelinAdvisor
M.Sc., Lic. Sc. (Tech.). Development Manager, Tampere University Hospital RDI center. Senior Advisor, Regulatory Affairs and author of “The Survival Guide to EU Medical Device Regulations”.

Kalle ÅströmVC, Advisor
M.Sc. (Econ.). Seasoned venture capital professional and business analyst. Representative of ERDF Investment Fund (EAKR-Aloitusrahasto).
